Searching for the centromere: diversity in pathways key for cell division
A research team at The University of Osaka has found a novel pathway for depositing CENP-A into vertebrate centromeres, which is key for microtubule attachment and subsequent cell division
| Journal | Embo J. (2026) |
|---|---|
| Title | Dual pathways via CENP-C and Mis18C recruit HJURP for CENP-A deposition into vertebrate centromeres |
| Laboratory | Laboratory of Chromosome Biology〈Prof. FUKAGAWA Tatsuo〉 |
Despite the immense amount of genetic material present in each cell, around three billion base pairs in humans, this material needs to be accurately divided in two and allocated in equal quantities. The centromere, located in the middle of each chromosome, is known as the site where cellular equipment attaches to divide chromosomes successfully, but the specific mechanisms behind this remain unknown.
In a major new study reported in The EMBO Journal, researchers at The University of Osaka have identified an additional pathway by which the DNA-packaging histone CENP-A associates with and specifies the location of the centromere. This process is vital for ensuring chromosomes are structured and genes are expressed appropriately.
The study was based around the Holliday Junction Recognition Protein (HJURP), a so-called chaperone protein that ushers centromere-identifying cell components to the correct site on chromosomes. They found that HJURP did not localize at the centromere when the expression of both of two cell components, CENP-C and Mis18C, were eliminated following a double knockout process.
"Although it was known that Mis18C recognizes the chaperone HJURP to enable CENP-A's deposition onto centromeres, we found that CENP-C can actually occupy Mis18C’s role in this process, providing a parallel pathway that helps ensure successful and timely mitosis or meiosis," says senior author, Tatsuo Fukagawa. "We also identified the particular residues of HJURP that enable its binding to CENP-C."
The team then built on these findings through analyses in DT40 chicken cells, confirming that these interactions are essential for centromere function during cell division. These analyses showed that when HJURP and CENP-C did not interact, this led to errors in mitosis, slowing cell growth. The combination of no interaction and the removal of Mis18C meant that CENP-A could not be incorporated into chromatin, preventing cellular machinery from knowing the supposed location of the centromere.
"Our work reveals that this sequence-independent epigenetic mechanism of centromere specification has greater diversity than previously thought," explains lead author, Tetsuya Hori. "Given how biologically fundamental the processes of mitosis and meiosis are, our finding that the cell has independent pathways for flagging the location of each chromosome’s centromere is valuable."
The key findings of this work, regarding the existence of dual pathways for recruiting HJURP for CENP-A deposition, can provide a solid foundation for future studies on the mechanisms behind centromere functioning and on diseases involving errors of cell division.
Abstract
Centromere position is specified and maintained by sequence-independent epigenetic mechanisms in vertebrate cells, with the incorporation of the centromere-specific histone H3 variant CENP-A into chromatin being a key event for centromere specification. Although many models for CENP-A incorporation have been proposed, much remains unknown. In this study, we reveal that the CENP-A chaperone HJURP directly binds to the C-terminal domain of chicken CENP-C in vitro and that this interaction is essential for new CENP-A incorporation in chicken DT40 cells. While existing models have suggested that HJURP is recruited by the Mis18 complex (Mis18C), here, we propose that CENP-C and Mis18C provide dual recruitment pathways for HJURP localization to centromeres in DT40 cells. We demonstrate that both HJURP localization and new CENP-A incorporation are completely abolished in Mis18C knockout cells expressing an HJURP mutant lacking CENP-C binding ability. Furthermore, co-immunoprecipitation experiments reveal that CENP-C, HJURP and Mis18C form a tight association in the chromatin fraction. These two pathways are critical for robust CENP-A incorporation to maintain centromere position in vertebrate cells.
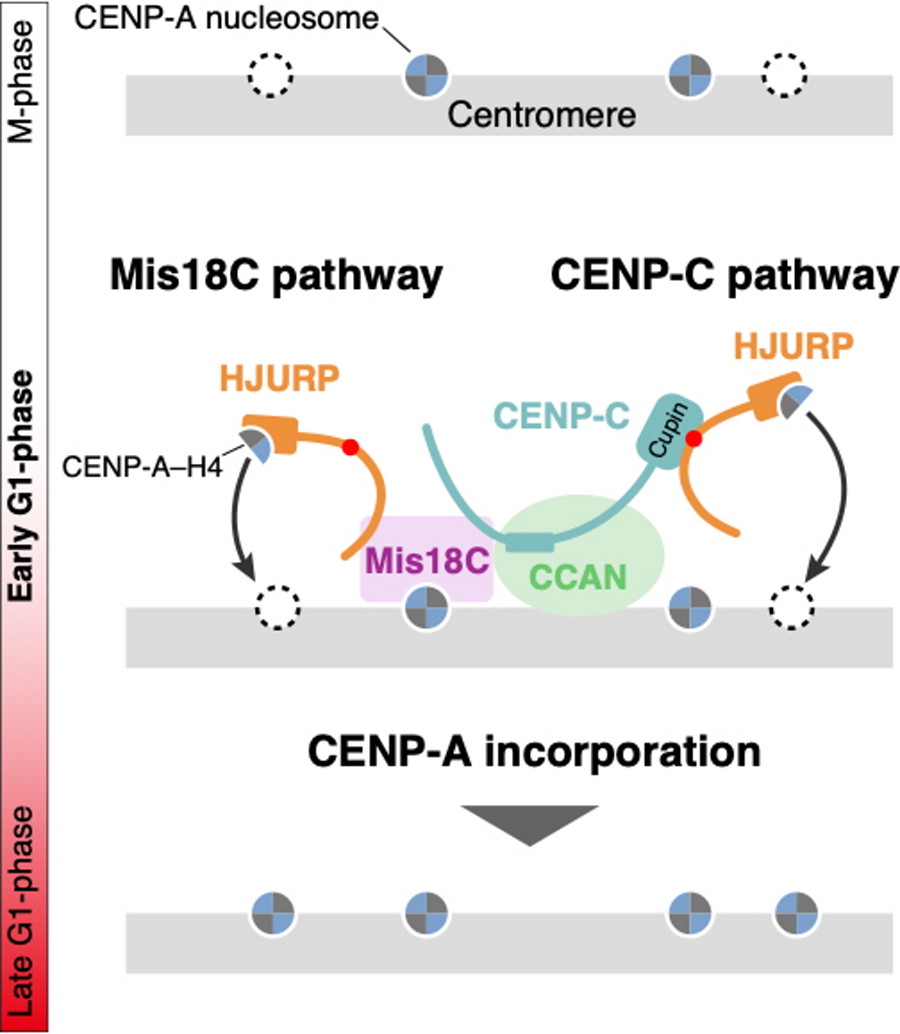
Fig. 1
In cells, CENP-A binds to histone H4 and HJURP before it is incorporated into the centromere. The previous model proposed that HJURP recognizes Mis18C and CENP-A is incorporated into the centromere during the G1 phase (Mis18C pathway). However, Hori et al. found that CENP-A is still incorporated even when the Mis18C pathway is inhibited. This suggests the existence of an additional pathway for CENP-A incorporation. Further analysis revealed that the CENP-A-H4-HJURP complex recognizes another centromere protein, CENP-C, and CENP-A is incorporated into the centromere (CENP-C pathway). Simultaneous inhibition of both pathways completely abolished CENP-A incorporation, resulting in cell death due to abnormal mitosis.
| Authors | Tetsuya Hori (1), Yutaka Mahana (1), Mariko Ariyoshi (1), Tatsuo Fukagawa (1)
|
|---|
