Cooperative motor proteins found to kill cancer cells when dual-inhibited
Discovery reveals how key molecules coordinate chromosome alignment and offers a new anticancer target
| Journal | Cell Reports, 116515 (2025) |
|---|---|
| Title | KIF18A promotes chromosome congression in cooperation with CENP-E downstream of CENP-C |
| Laboratory | Laboratory of Chromosome Biology〈Prof. FUKAGAWA Tatsuo〉 |
A research team from The University of Osaka, in collaboration with the Massachusetts Institute of Technology, has uncovered a new molecular mechanism underlying chromosome alignment during cell division. The study demonstrates that two motor proteins, KIF18A and CENP-E, act cooperatively to ensure proper chromosome congression. Remarkably, simultaneous inhibition of these proteins selectively kills cancer cells, suggesting a promising therapeutic avenue.
Accurate chromosome segregation is essential for healthy cell division; its failure leads to chromosomal instability—a hallmark of cancer. While the kinetochore, a protein complex on chromosomes, coordinates this process, the overlapping roles of many proteins have made it difficult to clarify the exact mechanisms.
Using a genome-wide CRISPR screen in cells with a mild defect of kinetochore (the chromosomal structure where sorting machinery attaches) by a CENP-C mutation, the researchers identified KIF18A as a gene whose loss caused synthetic lethality with the CENP-C mutation. Further analyses revealed that this kinetochore defect stemmed from reduced CENP-E activity, indicating that KIF18A and CENP-E cooperate downstream of CENP-C to drive early-stage chromosome alignment. Cancer cells with naturally low CENP-E levels were found to be highly sensitive to KIF18A inhibition, and combined inhibition of both proteins effectively induced cell death.
The research team used a unique cell line with a partially defective kinetochore to screen for genetic weaknesses. They found that these cells died when the KIF18A gene was disrupted. Further investigation revealed that these cells also had reduced levels of another motor protein, CENP-E.
The study demonstrated that KIF18A and CENP-E work together to ensure chromosomes align properly at the center of a cell. In normal cells, if one protein's function is compromised, the other can often compensate. However, when both are functionally impaired, chromosome alignment fails completely, leading to cell death. Crucially, the research team found that cancer cells sensitive to KIF18A inhibitors naturally have low levels of CENP-E, explaining why the drugs are so effective in these specific cases.
The study suggests that some cancer cells become especially vulnerable when they have low levels of a protein called CENP-E. This means that measuring CENP-E could help identify cancers that respond well to drugs blocking another protein, KIF18A. The results also hint that combining drugs that affect these two proteins might make cancer treatments more effective.
"We not only uncovered detailed mechanisms of chromosome segregation but also applied these findings to efficiently kill cancer cells, emphasizing that such targeted therapies must be grounded in basic research," says senior author Professor Tatsuo Fukagawa.
Abstract
Chromosome congression is a key process that acts to align chromosomes at the spindle equator via kinetochore-microtubule interactions, with defects in chromosome alignment leading to chromosomal instability. However, defining the mechanisms that underlie chromosome congression is limited due to the multiple factors that act in parallel to regulate chromosome movement. Here, we conducted a genome-wide Cas9-based functional genetics screen using a hypomorphic CENP-C mutant that affects its kinetochore interactions. Our analysis identified KIF18A, whose knockout resulted in synthetic lethality with the CENP-C mutant. Further analysis revealed that the synthetic defect was due to a reduction in CENP-E function in the CENP-C mutant. Our work suggests that KIF18A promotes chromosome alignment in cooperation with CENP-E downstream of CENP-C during early prometaphase. Thus, our analysis enables us to dissect parallel molecular mechanisms for chromosome congression and identify sensitivities and biomarkers that might guide anti- KIF18A chemotherapeutics.
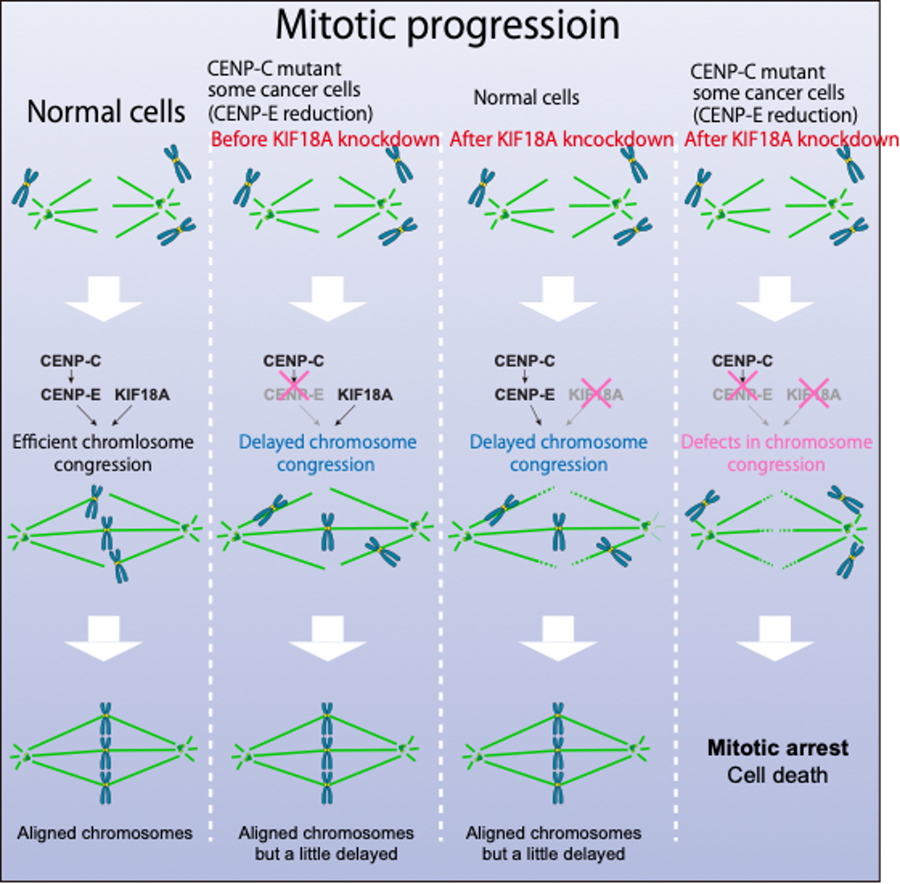
Fig. 1
Summary of this study. Left: Normal cells. Second from left: Cells with slightly reduced CENP-E function. These cells show slightly delayed chromosome alignment but remain viable before KIF18A knockdown. Third from left: Cells with normal function or sufficient CENP-E function. These cells do not die after KIF18A function is disrupted. Right: ells with slightly reduced CENP-E function. These cells die after KIF18A function is disrupted, because chromosome congression never happened.
| Authors | Jiahang Miao (1), Masatoshi Hara (1, 2), Kuan-Chung Su (3), Heather R. Keys (3), Weixia Kong (1), Yusuke Takenoshita (1), Iain M. Cheeseman (3, 4), Tatsuo Fukagawa (1)
|
|---|
