Ear today, gone tomorrow? A new discovery in a cause of inner-ear bone loss
| Journal | Nat. Commun. (2023) |
|---|---|
| Title | Single-cell transcriptomics of human cholesteatoma identifies an activin A-producing osteoclastogenic fibroblast subset inducing bone destruction |
| Laboratory | Laboratory of Immunology and Cell Biology〈Prof. ISHII Masaru〉 |
Chronic inflammation of the middle ear can cause several problems and complications that can affect a person’s hearing and balance. One such problem is the formation of a cholesteatoma, which is an abnormal collection of cells in the ear that can cause bone erosion if left untreated. In turn, this can cause symptoms such as hearing loss, dizziness, facial paralysis, and even a brain infection.
In a study published recently in Nature Communications, researchers from Osaka University have revealed the cause of cholesteatomas, which may help in developing new therapies for patients who are suffering from this disease.
Cholesteatomas are made up of cysts or bumps in the ear that consist of skin, collagen fibers, skin cells, fibroblasts, keratin, and dead tissue. There are many theories on how these cholesteatomas can cause bone erosion, including the activation of cells responsible for the breakdown of the minerals and matrix of the bone, the presence of inflammatory markers and enzymes, and the accumulation and pressure from dead cells and tissues in the ear; however, the exact mechanism for the creation of cholesteatomas remains unknown. “A cholesteatoma can still return or happen again even after its surgical removal, so it is important to know what is actually causing it,” says lead author Kotaro Shimizu.
To investigate this, researchers looked at human cholesteatoma tissues that were surgically removed from patients. A process called single-cell RNA sequencing analysis was employed to identify cells responsible for triggering bone erosion; these were called osteoclastogenic fibroblasts. This study demonstrated how these fibroblasts expressed an abundant amount of activin A, a molecule that regulates different physiologic functions of the body. The presence of activin A is said to cause bone erosion through a process in which specialized cells initiate bone resorption through a process wherein the minerals and matrix of the bones are broken down and absorbed by the body.
The researchers were successful in showing the relationship between activin A and bone erosion in cholesteatoma. “Our study showed that targeting activin A is a potential treatment in the management of cholesteatomas,” states senior author Masaru Ishii.
Currently in clinical settings, the only effective treatment for cholesteatomas is complete surgical removal. However, the discovery of how a cholesteatoma can cause bone erosion in this study offers new hope for developing novel medical treatments as first-line management for cholesteatomas.
Abstract
Cholesteatoma, which potentially results from tympanic membrane retraction, is characterized by intractable local bone erosion and subsequent hearing loss and brain abscess formation. However, the pathophysiological mechanisms underlying bone destruction remain elusive. Here, we performed a single-cell RNA sequencing analysis on human cholesteatoma samples and identify a pathogenic fibroblast subset characterized by abundant expression of inhibin βA. We demonstrate that activin A, a homodimer of inhibin βA, promotes osteoclast differentiation. Furthermore, the deletion of inhibin βA /activin A in these fibroblasts results in decreased osteoclast differentiation in a murine model of cholesteatoma. Moreover, follistatin, an antagonist of activin A, reduces osteoclastogenesis and resultant bone erosion in cholesteatoma. Collectively, these findings indicate that unique activin A-producing fibroblasts present in human cholesteatoma tissues are accountable for bone destruction via the induction of local osteoclastogenesis, suggesting a potential therapeutic target.
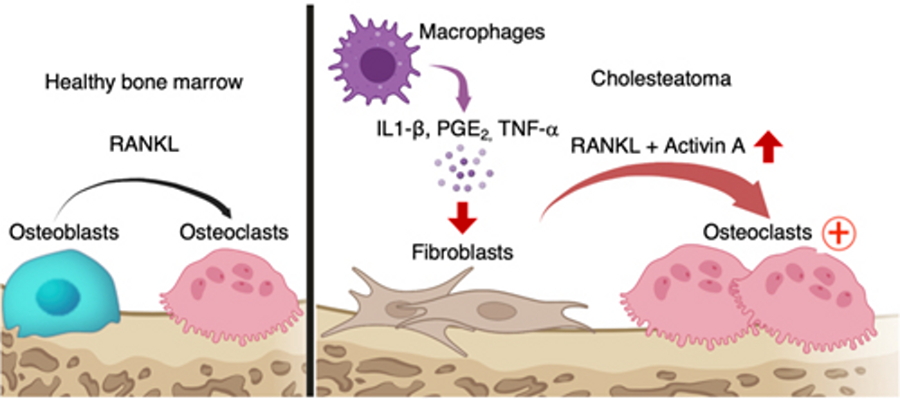
Schematic of osteoclastogenesis induced by cholesteatoma fibroblasts expressing activin A. IL-1β, PGE2, and TNF-α secreted from infiltrating CD45+ cells, particularly macrophages, induced activin A-expressing pathogenic fibroblasts; the activin A acted in conjunction with RANKL to promote ectopic osteoclastogenesis.
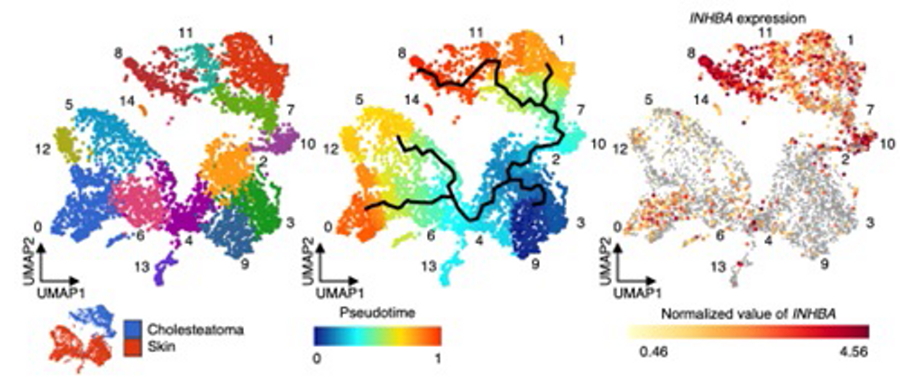
Subclustering and pseudotime analysis of human cholesteatoma fibroblasts. Cholesteatoma fibroblasts were associated with five subclusters labeled 1, 7, 8, 10, and 11 (right panel). The most differentiated cells (labeled red) were identical to cholesteatoma fibroblasts in subcluster 8 (middle panel). Cholesteatoma fibroblasts showed high levels of INHBA expression, and the area of high INHBA expression was identical to the area in cholesteatoma fibroblasts in subcluster 8 (left panel).
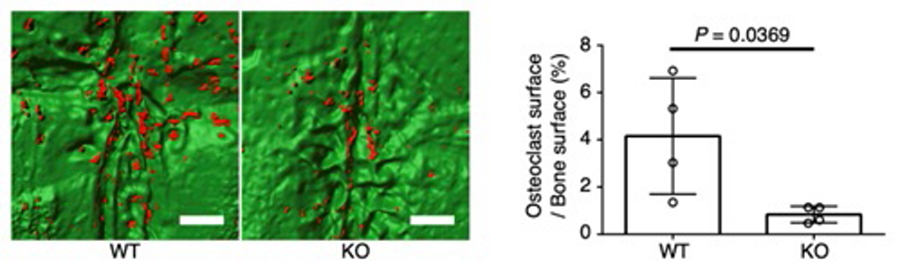
The in vivo role of activin A in osteoclastogenesis in an experimental mouse model of cholesteatoma. INHBA inhibition in fibroblasts reduced osteoclast formation on the parietal bone surface under the cholesteatoma mass. Red, osteoclasts; green, parietal bone surface. Scale bars: 500 µm.
| Authors | Kotaro Shimizu (1, 2, 3), Junichi Kikuta (1, 2, 4), Yumi Ohta (3), Yutaka Uchida (1, 2), Yu Miyamoto (1, 2), Akito Morimoto (1, 2), Shinya Yari (1, 2), Takashi Sato (3), Takefumi Kamakura (3), KazuoOshima (3), Ryusuke Imai (3), Yu-Chen Liu (5, 6), Daisuke Okuzaki (5, 6), Tetsuya Hara (7), Daisuke Motooka (5, 6), Noriaki Emoto (7), Hidenori Inohara (3), Masaru Ishii (1, 2, 4)
|
|---|
