Getting to the "Heart" of Heart Beats: Cardiac thin filament structure and function revealed
| Journal | Nat. Commun. 11(1):153 (2020) |
|---|---|
| Title | Getting to the "Heart" of Heart Beats: Cardiac thin filament structure and function revealed |
| Laboratory | JEOL YOKOGUSHI Research Alliance Laboratories〈SA Prof. NAMBA Keiichi〉 |
Researchers at Osaka University used electron cryomicroscopy (CryoEM) to image essential cardiac muscle components, known as thin filaments, with unprecedented resolution. They also discovered the mechanism by which these filaments regulate heart beat via cardiac muscle contractions in the presence or absence of calcium ions by changing their conformations. This work may have application in the development of new drugs for treating heart conditions caused by mutations that affect these structures and functions.The human heart is a remarkable organ, capable of pumping blood for a lifetime without rest. However, many of the details of its inner workings remain unknown, partly because the exact structures of its muscle proteins in their natural forms are difficult to image. This is especially true for "thin filaments" - tiny filamentous structures made up of proteins called actin, troponin, and tropomyosin - owing to their complex interactions and small size. It has long been known that cardiac muscle contraction is controlled by the repeated increase and decrease in the concentration of calcium ions within muscle cells and that the control of contraction is accomplished by changes in the structure of the thin filaments when these ions bind to them. However, the exact mechanism was unclear.Now, by using CryoEM, a technique recognized with the 2017 Nobel Prize in Chemistry, researchers at Osaka University have revealed the highest-resolution structural images of these proteins to date. Conventional electron microscopy usually damages fragile biological samples, meaning that their native shape in the body cannot be determined. In contrast, by the cryoEM technique, samples are flash-frozen so that proteins can be imaged while still in their native conformations."It has been very difficult to reveal the entire structure of the thin filament, but we succeeded in solving its structure using cryoEM and advanced image analysis," says first author Yurika Yamada. The Osaka team demonstrated how, in the absence of calcium ions, myosin access to the actin regions are blocked so that myosin heads cannot attach to them for muscle contraction. However, the binding of calcium ion changes the conformation of the thin filaments, exposing the attachment sites for contraction."Since many mutations in the component proteins of the thin filament are known to cause heart disease, including cardiac hypertrophy and cardiomyopathy, the revealed structures could provide a molecular and structural basis for novel drug design," explain senior authors Takashi Fujii and Keiichi Namba.This research also highlights the power of cryoEM to reveal previously unseen anatomical detail with potential for yet unimagined medical breakthroughs.
Abstract
Contraction of striated muscles is driven by cyclic interactions of myosin head projecting from the thick filament with actin filament and is regulated by Ca2+ released from sarco- plasmic reticulum. Muscle thin filament consists of actin, tropomyosin and troponin, and Ca2+ binding to troponin triggers conformational changes of troponin and tropomyosin to allow actin-myosin interactions. However, the structural changes involved in this regulatory mechanism remain unknown. Here we report the structures of human cardiac muscle thin filament in the absence and presence of Ca2+ by electron cryomicroscopy. Molecular models in the two states built based on available crystal structures reveal the structures of a C-terminal region of troponin I and an N-terminal region of troponin T in complex with the head-to-tail junction of tropomyosin together with the troponin core on actin filament. Structural changes of the thin filament upon Ca2+ binding now reveal the mechanism of Ca2+ regulation of muscle contraction.
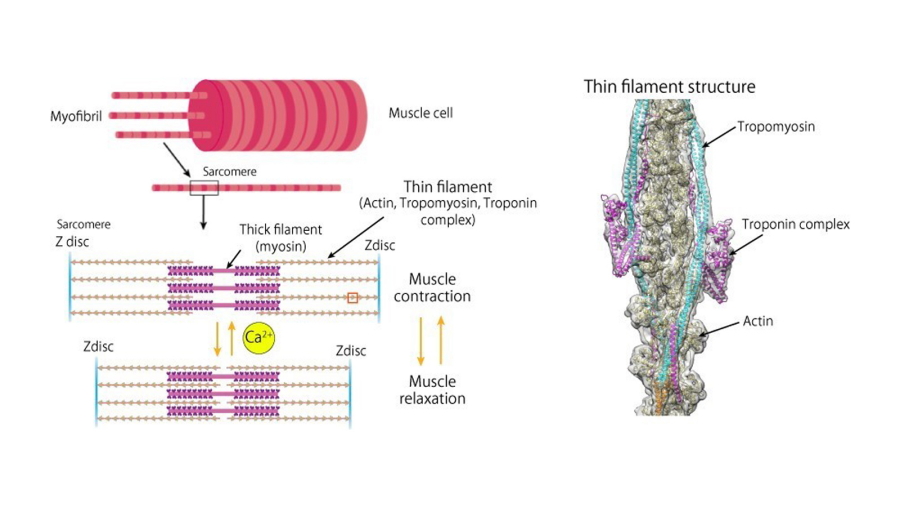
Fig. 1
Schematic diagram of the muscle fiber structure (left) and the molecular structure of the entire thin filament (right).
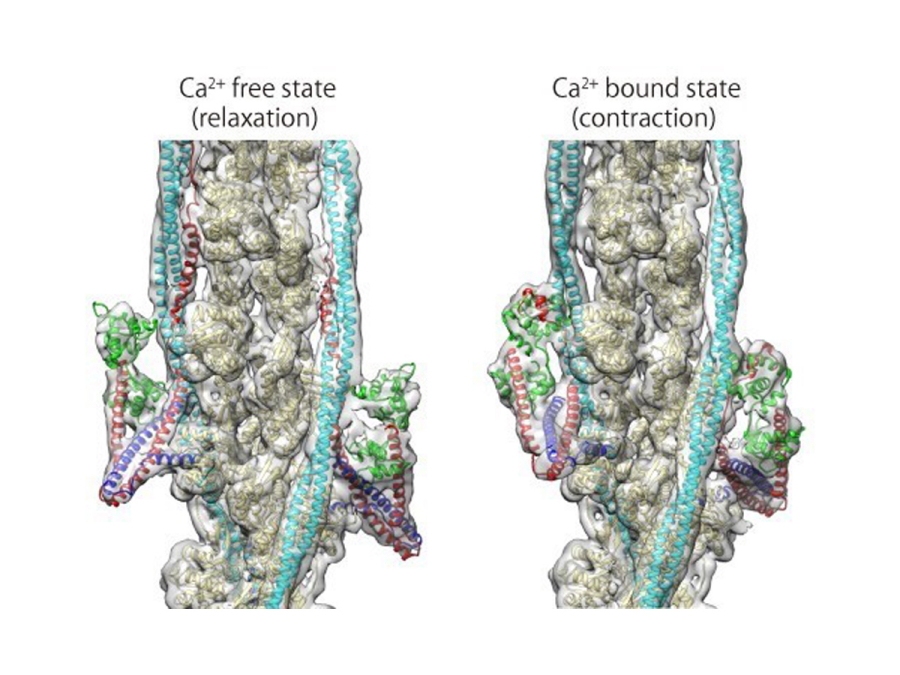
Fig. 2
Structures of the thin filament in the absence (left) and presence (right) of Ca2+.
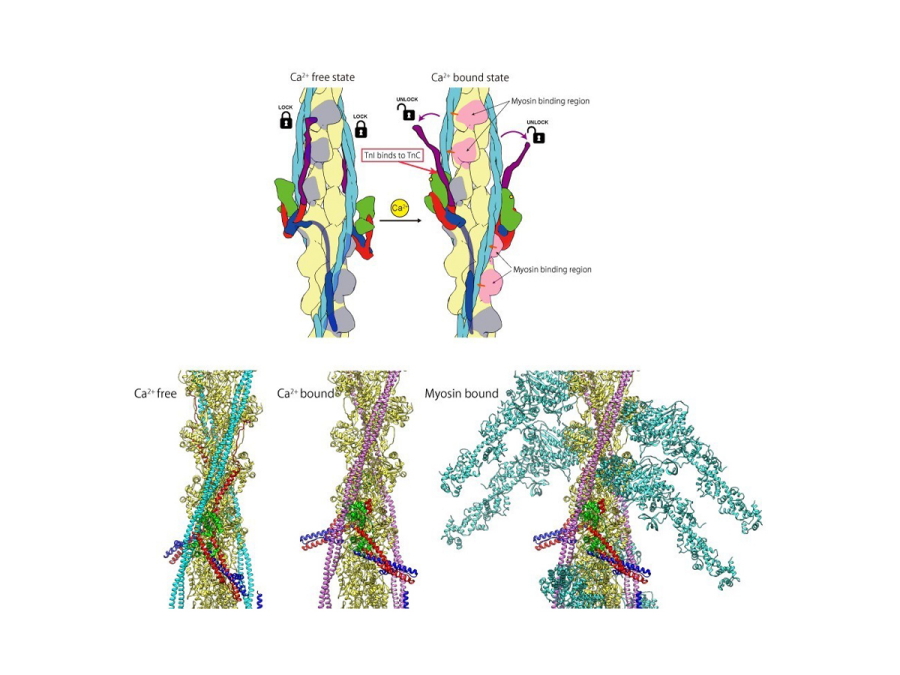
Fig. 3
Schematic diagram of the structural change of the thin filament upon Ca2+ binding to troponin C (upper panel) and three structural models of the thin filament (lower panel) including the myosin head bound state (right).
| Authors | Yurika Yamada (1), Keiichi Namba (1, 2, 3) , Takashi Fujii (1, 3)
|
|---|---|
| PubMed | 31919429 |
