Structure of the native supercoiled flagellar hook as a universal joint
| Journal | Nat. Commun. 10(1):5295 (2019) |
|---|---|
| Title | Structure of the native supercoiled flagellar hook as a universal joint |
| Laboratory | JEOL YOKOGUSHI Research Alliance Laboratories〈SA Prof. NAMBA Keiichi〉 |
Abstract
The Bacterial flagellar hook is a short supercoiled tubular structure made from a helical assembly of the hook protein FlgE. The hook acts as a universal joint that connects the flagellar basal body and filament, and smoothly transmits torque generated by the rotary motor to the helical filament propeller. In peritrichously flagellated bacteria, the hook allows the filaments to form a bundle behind the cell for swimming, and for the bundle to fall apart for tumbling. Here we report a native supercoiled hook structure at 3.6Å resolution by cryoEM single particle image analysis of the polyhook. The atomic model built into the three-dimensional (3D) density map reveals the changes in subunit conformation and intersubunit interactions that occur upon compression and extension of the 11 protofilaments during their smoke ring-like rotation. These observations reveal how the hook functions as a dynamic molecular universal joint with high bending flexibility and twisting rigidity.
- We have revealed the mechanism of molecular universal joint that smoothly transmits torque of the rotary motor to the helical propeller of the bacterial flagellum.
- It was thought impossible to analyze the structure of supercoiled hook in its functional state, but we succeeded in high-resolution structural analysis using cryoEM single particle analysis.
- Bacterial motility is deeply involved in pathogenicity, and the revealed structure leads not only to development of new antibiotics but also to design of nanodevices.
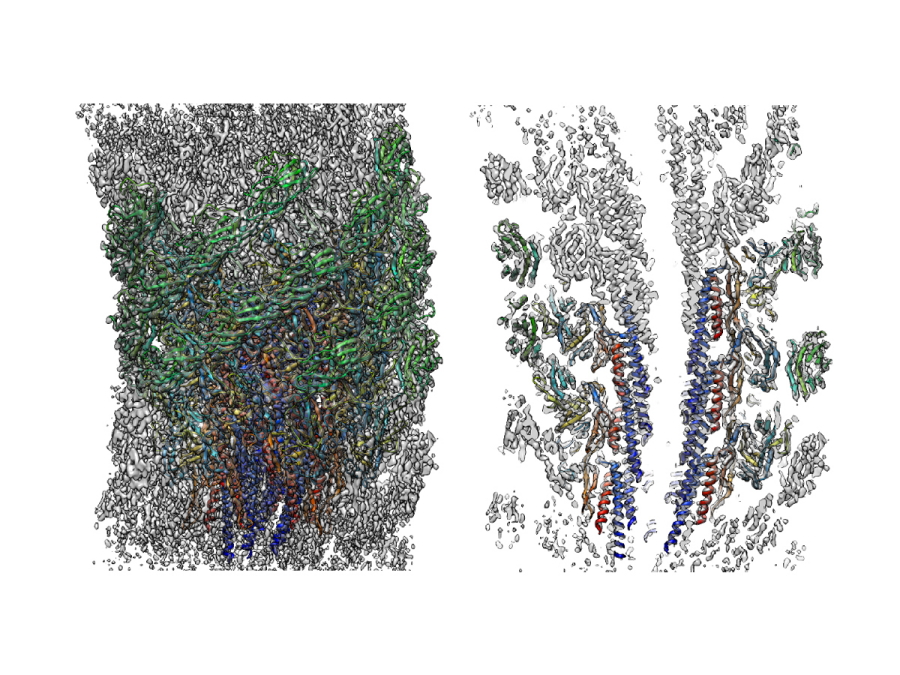
Fig. 1
Three dimensional density map and atomic model of the supercoiled flagellar hook revealed by cryoEM image analysis. Left: side view; right: a central section.
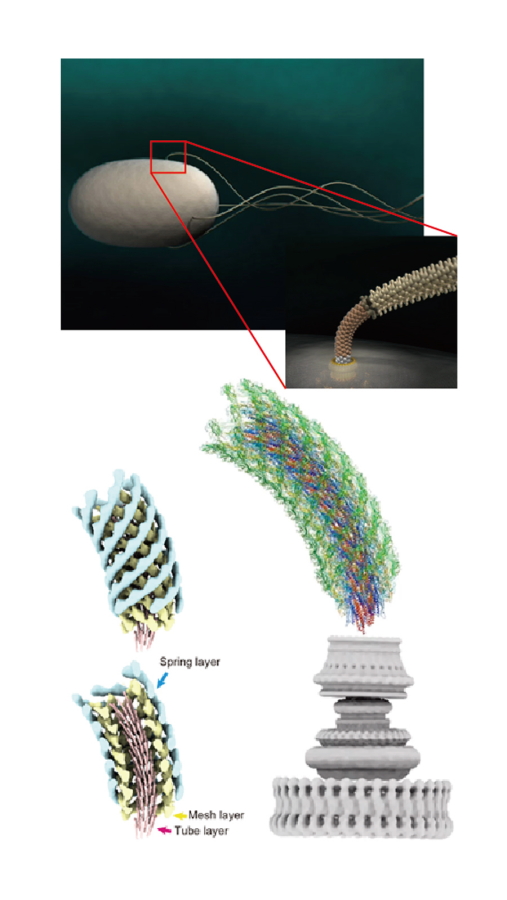
Fig. 2
Computer graphic representation of the bacterial cell and flagella (top left) and its hook connecting the motor and filament (top right). Three-layered tube model of the hook structure (bottom left) and atomic model of the entire hook on top of the flagellar motor (bottom right).
| Authors | Takayuki Kato (1, 2), Fumiaki Makino (1, 3), Tomoko Miyata (1), Péter Horváth (1, 4), Keiichi Namba (1, 3, 5)
|
|---|---|
| PubMed | 31757961 |
