High-tech tracking technology streamlines drug discovery
| Journal | Nat. Commun. 15(1):8975 (2024) |
|---|---|
| Title | Single molecule tracking based drug screening |
| Laboratory | Laboratory of Single Molecule Biology〈Prof. UEDA Masahiro〉 |
New drug discovery is a critical step for improving patients’ lives. First, researchers must identify molecules in the body’s cells that help drive disease as these are potential targets for new drugs. The next step is to screen candidate drugs that can hit those targets. But screening can be a challenging and time-consuming process.
In a new study published in Nature Communications, a team at Osaka University has developed a technology that streamlines drug discovery using single-molecule tracking. This method lets the researchers explore the effects of many different candidate drugs on a single target molecule. Building on the team’s large-scale intracellular single molecule imaging system (AiSIS), the technology screens new drugs 100 times faster than standard manual techniques. The team tested their new method to screen drugs that target the epidermal growth factor receptor (EGFR), a molecule central to the development and progression of various cancers. This was a good way to check how well their screening worked because several drugs that block the EGF receptor are already available to treat lung cancer.
"We used a library of over 1,000 approved drugs to test our screening method," says lead author of the study Daisuke Watanabe. "We successfully identified all the drugs that are known to target the EGFR and are currently used to treat cancer patients. More importantly, we found that the library included seven drugs that until now were not known to affect the EGFR."
The new imaging technique visualizes the behavior of the EGFR after treatment with each drug, allowing the researchers to see how it reacted. For example, it’s now possible to see changes in the assembly and disassembly of target molecules in response to drug treatment, a process known as multimer formation.
"Screening using single-molecule imaging provides a new means to discover drugs by observing the movement of biomolecules in cells and the formation of multimers," explains senior author Masahiro Ueda. "This has not been used for drug discovery until now, and it means we should be able to develop new drugs with different mechanisms of action and even repurpose already approved drugs to new targets."
The study has proven that the researchers’ method works using the well-known EGFR target, they can focus on screening drugs that could hit an array of other receptor targets that are closely involved in disease development and progression.
Abstract
The single-molecule tracking of transmembrane receptors in living cells has provided significant insights into signaling mechanisms, such as mobility and clustering upon their activation/inactivation, making it a potential screening method for drug discovery. Here we show that single-molecule tracking-based screening can be used to explore compounds both detectable and undetectable by conventional methods for disease-related receptors. Using an automated system for a fast large-scale single-molecule analysis, we screen for epidermal growth factor receptor (EGFR) from 1134 of FDA approved drugs. The 18 hit compounds include all EGFR-targeted tyrosine kinase inhibitors (TKIs) in the library that suppress any phosphorylation-dependent mobility shift of EGFR, proving the concept of this approach. The remaining hit compounds are not reported as EGFR-targeted drugs and do not inhibit EGF-induced EGFR phosphorylation. These non-TKI compounds affect the mobility and/or clustering of EGFR without EGF and induce EGFR internalization, to impede EGFR-dependent cell growth. Thus, single-molecule tracking provides an alternative modality for discovering therapeutics on various receptor functions with previously untargeted mechanisms.
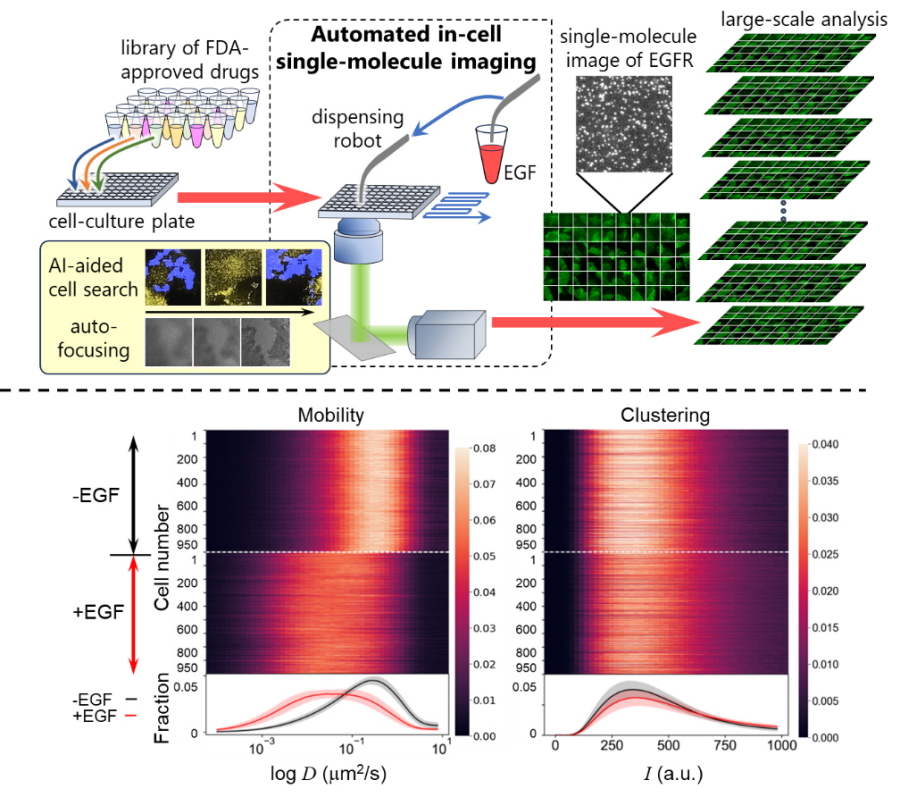
Fig. 1
Top) Drug screening with automated system for in-cell single-molecule imaging. Bottom) Diffusion coefficient of the receptor (left) and cluster formation (right) were measured from 1,000 cells. These distributions obtained by every cell changed with EGF stimulation.
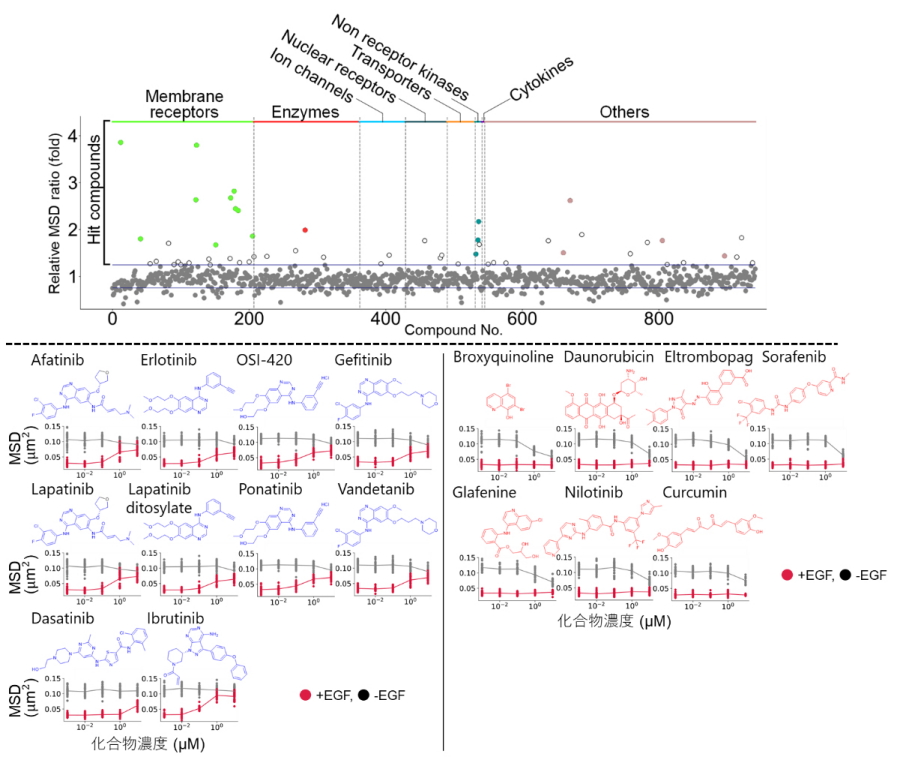
Fig. 2
Top) Library used for the screening. Color dots indicate the selected drugs. Bottom) 10 TKIs (left) and other 7 drugs (right) are included. MSD denotes mean square displacement of receptor diffusion.

Fig. 3
Three different mechanisms of action for drugs effective for EGFR which were selected by single-molecule tracking-based screening.
| Authors | Daisuke Watanabe (1, 2), Michio Hiroshima (1, 2), Masato Yasui (3), Masahiro Ueda (1, 2, 4)
|
|---|---|
| PubMed | 39420015 |
