Professor Noriyuki Tsumaki's 'The Diverging Path, The Continuing Path' Part 1
Applying the science
2023.10.17

Meet Professor Noriyuki Tsumaki, a distinguished figure in the field of Tissue Biochemistry Research. With a strong focTus on regenerative medicine, he has harnessed the power of iPS cell technology to pioneer the clinical restoration of knee joint cartilage in patients. In 2023, his groundbreaking work ventured into the use of primate models to delve into the intricacies of regenerating mechanisms for homologous cartilage transplantation. This research serves as a cornerstone for advancements in the field.
In 2022, Professor Tsumaki achieved yet another milestone by developing a novel approach to regenerate intervertebral discs, leveraging a rat model for the purpose. But his journey is not just about scientific breakthroughs; it's about the intersection of science and its real-world impact.
As a practicing orthopedic surgeon, Professor Tsumaki brings a unique perspective to his research, ensuring that it aligns seamlessly with clinical applications. We sat down with him to explore his reflections on the synergy between science and practical applications, his personal journey as a researcher, and the dynamic operation of his cutting-edge research laboratory.
I didn't understand what research was
I didn't plan to become a researcher
When I graduated from university, I didn't plan to become a researcher. Many students who graduate from medical school go on to become doctors, and I was no exception. However, as I observed my seniors, I learned that there was a path to enter graduate school after about three years of training as a doctor (a medical resident) and then pursue research. This is when I decided that I wanted to go to graduate school and engage in research.
Over those three years, as I was treating various conditions (including bone, cartilage, and peripheral nerve-related issues), I found myself increasingly intrigued by cartilage. While bone injuries, even complex fractures, often heal with the right immobilization or surgical intervention, cartilage presents a different challenge. It's a well-known fact in the field of orthopedics that once cartilage is injured, it doesn't heal, and this leaves a substantial number of patients in distress.
The fundamental lack of a curative method for cartilage injuries has led to a sense of perplexity within the medical community, as we grapple with the question of how to address these issues effectively. Consequently, it's no surprise that within the field of orthopedics, there is a growing community of researchers dedicated to the study of cartilage. It's a field that presents numerous challenges and a wealth of research opportunities, making it a rewarding and vital area of focus.
Cartilage doesn't heal when injured
Cartilage is not a one-size-fits-all entity; there are two primary types: articular cartilage, which is crucial for joint movement, and growth cartilage, responsible for bone elongation. This is the reason children grow taller. Growth cartilage typically disappears in the late teens, marking the end of bone growth. In contrast, articular cartilage remains throughout a person's lifetime. Unfortunately, it doesn't heal when injured. As a result of injuries and age-related changes, a significant number of patients suffer from degenerative joint diseases, such as osteoarthritis, where articular cartilage deteriorates.
One treatment option for such cases is joint replacement surgery. This procedure involves removing damaged cartilage and bone and replacing them with metallic components.
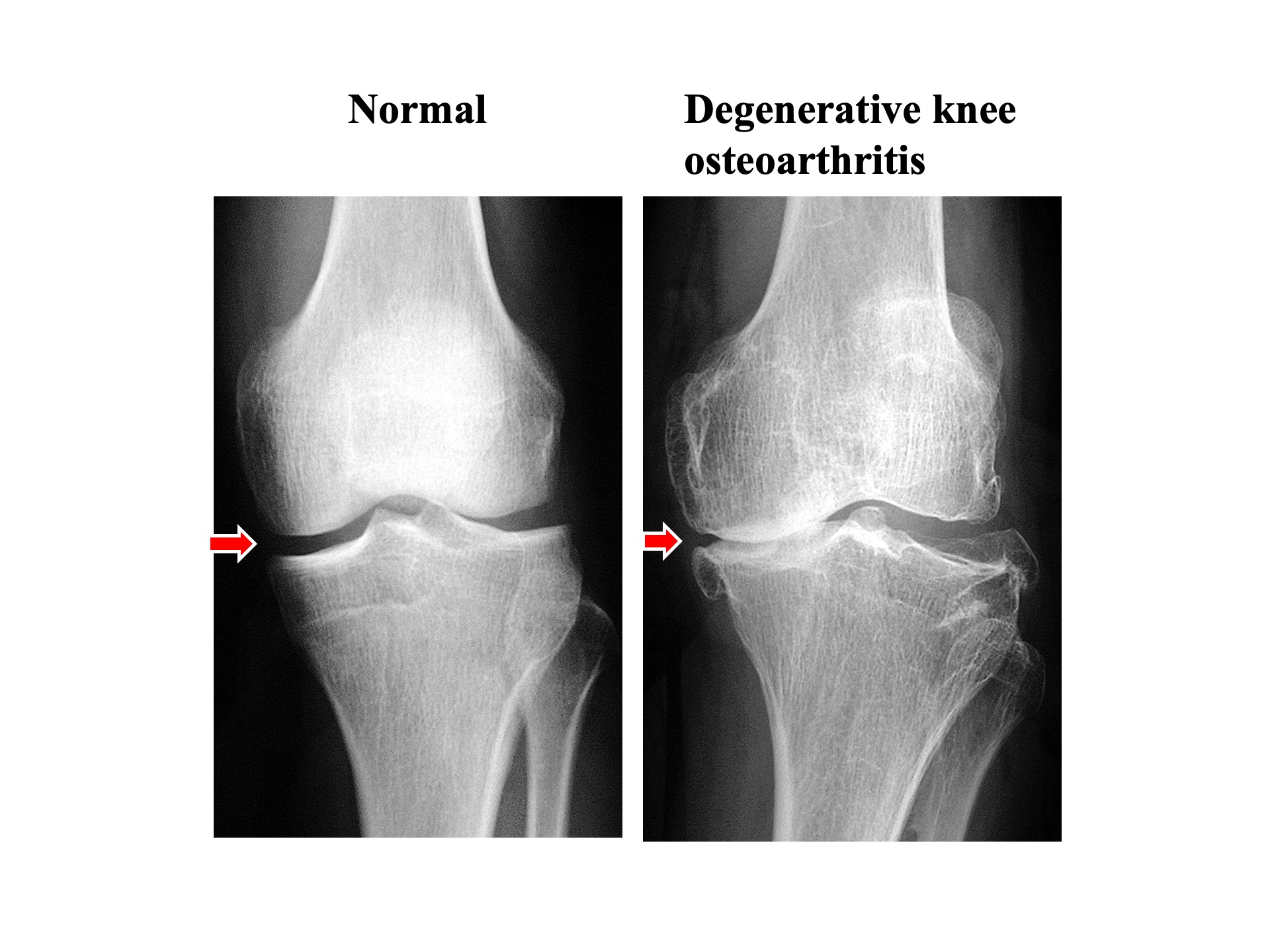
Degenerative knee osteoarthritis due to damage and loss of articular cartilage. Joints are crucial for smooth movement as they are covered by cartilage at the ends of the thigh bone and shin bone. Accidents, sports injuries, or the natural aging process can lead to cartilage damage, which, if left untreated, can progress into degenerative joint disease. While bones show up as white on X-rays, cartilage does not. Therefore, under normal conditions, you can observe cartilage as a gap between the bones (indicated by arrows). In degenerative joint disease, the reduction in cartilage leads to a narrowing of this gap (indicated by arrows).
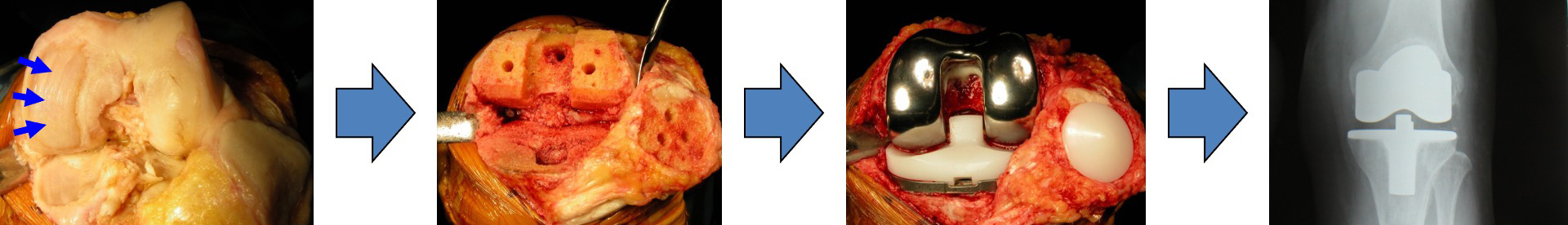
Advanced joint cartilage damage: total knee replacement
Surgery to replace knee or hip joints with artificial ones is typically reserved for patients in the advanced stages of joint damage. After such surgery, patients often find it challenging to engage in activities like running or jumping. Sitting cross-legged becomes difficult, and it can significantly restrict one's daily life. Consequently, joint replacement surgery is generally recommended for patients in the advanced stages of degenerative joint disease. Patients in the early stages of the condition usually don't experience significant benefits from artificial joint replacement.
The period from cartilage damage to its deterioration into the advanced stage can be quite lengthy, but regrettably, there is no definitive cure during this time. When the pain is manageable, conservative treatments like rest and physical therapy are often recommended. Many patients fall into this category.
I used to believe that the goal of medicine was to restore the body anatomically and heal it. However, I came to realize that this is exceptionally challenging with cartilage. In reality, surgery to replace the joint with an artificial one in the advanced stage is often the only viable option. This is a realization I came to after becoming a physician.
'In for a penny, in for a pound'
Before I entered graduate school, I honestly didn't have a clear understanding of what research entailed. I didn't have a specific vision in mind. It was when I learned about the impressive work being carried out by Professor Tomoatsu Kimura*, who specialized in cartilage research, that I decided I wanted to focus on cartilage under his guidance. That's what motivated me to enroll in graduate school.
I graduated from Osaka University Medical School in 1989, back when the campus was still at Nakanoshima**. I spent my first year of graduate school there and then moved to this campus in Suita in my second or third year, around 1993 or 1994. I was struck by the abundance of foundational research labs here, each working on cutting-edge and challenging research.
At that time, very few orthopedic surgeons were delving into molecular biology, except for Professor Kimura. So, being able to learn from that professor was a stroke of luck. What I learned from Professor Kimura has stayed with me to this day. The knowledge he shared on cartilage embryology and molecular biology significantly influences the work I'm doing now.
Professor Kimura often quoted this Japanese proverb, 'If you're going to eat poison, you may as well eat the plate as well', equivalent to the saying "In for a penny, in for a pound." In research, the outcome is uncertain, but when confronted with challenges, one should persevere and see the research through to completion without giving up midway. I believe that's the essence of what he meant.
- *Professor Tomoatsu Kimura, who graduated from Osaka University Medical School in 1978, played a pivotal role in Professor Noriyuki Tsumaki’s journey. During his time as an orthopedic lecturer, he mentored Professor Noriyuki Tsumaki when he was a graduate student. Subsequently, he went on to become a professor in orthopedics at Toyama Medical and Pharmaceutical University (now known as Toyama University).
- **Nakanoshima served as the campus for Osaka University Medical School and its affiliated hospital until 1992 when they relocated to Suita City. Professor Noriyuki Tsumaki graduated from Osaka University Medical School in 1989. During his time there, he was a member of the medical school's rugby team, and today, he serves as the team's advisor. The team is currently recruiting new team members.
Embarking on basic research without a specific focus on its clinical applications
When I entered the field of orthopedics, I noticed that there weren't many people who pursued basic research. At the beginning, my motivation for research was not centered around making it directly beneficial to clinical practice. Rather, it was because I had a genuine passion for science. At that time, I understood that basic research, in itself, might not immediately have a direct clinical impact or provide quick and straightforward solutions for clinical needs. That's when I decided to focus on research without the primary goal of immediate clinical application.
I've continued with this foundational research throughout my career. It's necessary to stay at the cutting edge and keep up with the latest developments. In clinical application, much effort is often dedicated to complying with regulations and standards, and this can sometimes shift the focus away from pure scientific advancement. As a result, those who primarily focus on clinical application may find themselves trailing behind in the forefront of research.
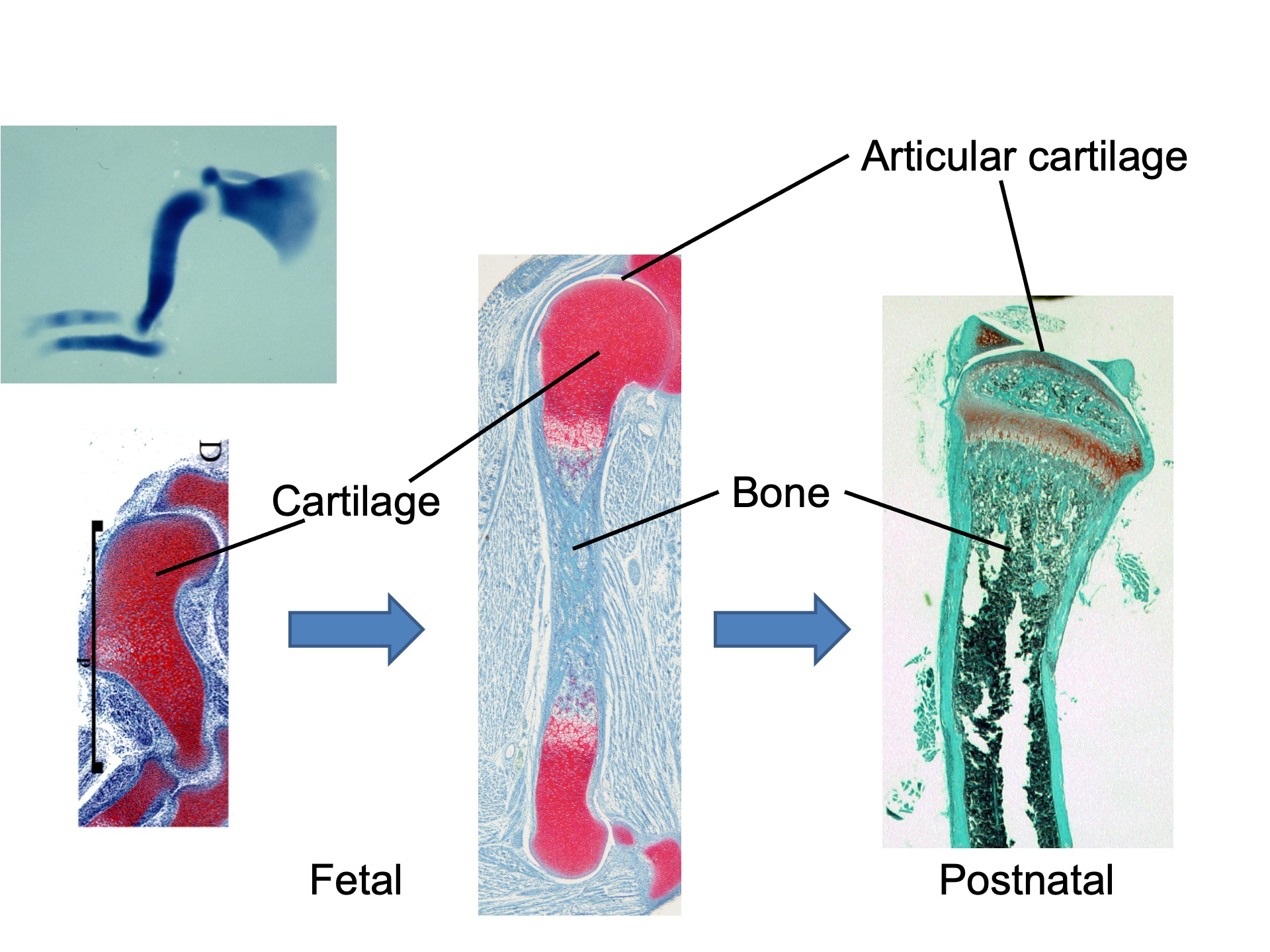
Cartilage Development and Skeletal Formation. Cartilage forms as a mass (the primordium of the skeleton) during fetal development, and with time, it gradually transforms into bone from the inside. The cartilage at the ends remains as articular cartilage, covering the bones and forming the joints.
What's really fascinating is the process of development. In animals, cartilage initially forms and then transforms into bone. The fact that cartilage forms is a remarkable occurrence in itself, and understanding how this process unfolds has always intrigued me.
Like any other organ, cartilage develops from the fertilized egg to the fetal stage. But the key to how this unique shape comes about lies in the control of gene expression. It's incredible to realize that just a single base pair difference in the DNA sequence of a gene can lead to significant changes in cartilage, sometimes resulting in diseases. It was during this type of research that I felt a sense of wonder.
I delved into studying the crucial molecules in the cartilage development process, how they interact, and the role of various molecules. By modifying certain genes in animal models, we could observe how cartilage took on different forms. I've been engaged in this kind of research for quite some time.
My first taste of success!
My first research paper* involved the gene cloning of type XI collagen** alpha 2 chain and the discovery of selective splicing*** in that gene. We screened a genomic DNA library to pick up phage clones containing the XI collagen gene. The sequences in those clones were around 10,000 base pairs long, so we had to subclone them and sequence tirelessly. We created sequencing gels and conducted electrophoresis repeatedly.
At that time, we could read a maximum of about 300 bases at once. When the primers weren't working well, we had to repeat the process of subcloning fragments into plasmids and sequencing. It was a challenging but rewarding experience.
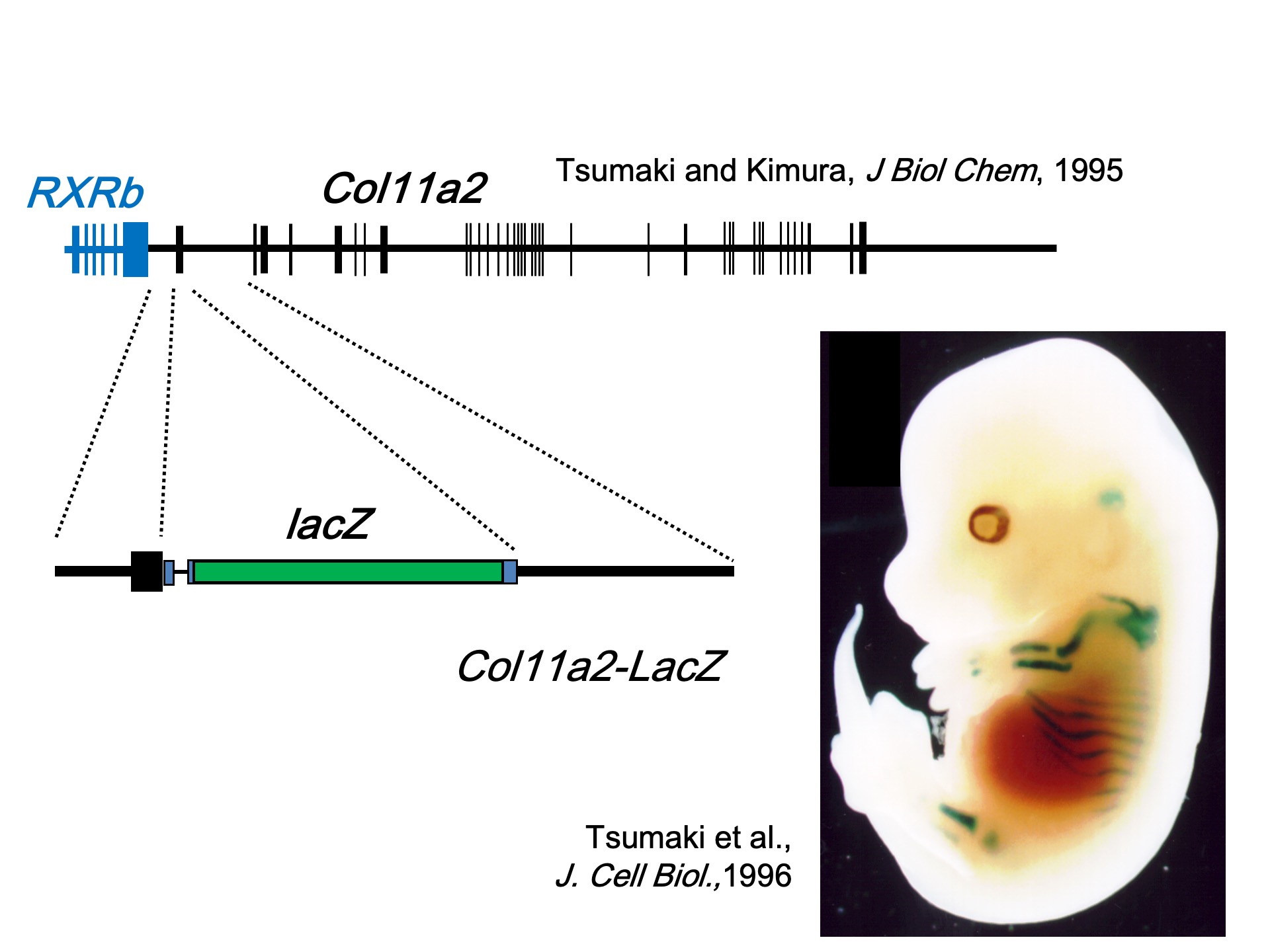
The cloning of the Col11a2 gene (Type XI collagen alpha 2 chain) and the identification of its promoter and enhancer sequences were pivotal. In transgenic mice, where a reporter gene lacZ was linked to the sequences (promoter and enhancer) necessary for the expression of the XI collagen gene in cartilage, lacZ expression was observed specifically in the cartilage primordia of the limbs and ribcage.
However, during the course of my experiments, I realized that the sequences we were working on were completely unknown to the rest of the world. It was a rather exciting feeling, knowing that I might be the first person in the world doing this. But at the same time, I also felt the pressure to finish quickly and publish a paper, as it was possible that someone somewhere else in the world was working on the same thing. In fact, we managed to publish a paper in the Journal of Biological Chemistry (JBC)****, and about six months later, a paper with the same sequence came out from a well-established lab in the United States, also in JBC. It was a realization that what Professor Kimura had been advising was indeed correct. This was my first taste of personal success.
- *Differential Expression of an Acidic Domain in the Amino-terminal Propeptide of Mouse Pro-α2(XI) Collagen by Complex Alternative Splicing (https://www.sciencedirect.com/science/article/pii/S0021925819480046?via%3Dihub)
- **It's the 11th of approximately 29 known types of collagen in the human body. It, along with Type II and Type IX collagen, forms collagen microfibers in the extracellular matrix of cartilage.
- ***In the transcription process from DNA, multiple messenger RNAs are produced from a single gene.
- ****A core journal in the fields of biochemistry and molecular biology.
The wisdom of my mentor in America: 'Something's bound to happen'
For some reason, we couldn't get any mice to reproduce
When I entered graduate school, one of my immediate thoughts was that I wanted to study abroad. I had this desire to experience living overseas. At that time, I was only thinking about the next two to three years. I particularly wanted to go to the United States, so I pursued a research fellowship at the National Institutes of Health (NIH). My mentor there was Dr. Yoshihiko Yamada*. Dr. Yamada was the first to successfully clone collagen genes. I had previously met him at an international connective tissue conference and had the opportunity to attend his lecture. Following Professor Kimura's recommendation, I went to study under Dr. Yamada, who was an expert in molecular biology, and he guided me in approaching basic research.
While I was in graduate school in Japan, I was part of a clinical research lab in the field of orthopedics. However, when I went to the United States, I joined a basic research lab, which was quite different. Well, Japan does have a bit of a strict hierarchical structure. It seems like clinical research labs, in particular, have that sort of hierarchy. In contrast, the United States is quite the opposite – they don't have that at all. Also, in the United States, lab bench space is notably more spacious. However, I did face some challenges with setting up experiments, and for a while, I couldn't obtain any data. It took about a year before I started getting results.
I was conducting experiments involving the creation of transgenic mice. This entailed using a microscope to guide a manipulator to pierce the nucleus of a fertilized egg with a needle, introducing DNA solution, and then returning the fertilized egg to the fallopian tube of a pregnant mouse to produce genetically modified mice. I had experience doing this back when I was a graduate student in Japan. However, for some reason, in the United States, the mice simply wouldn't reproduce. It took about a year before we finally saw some success. Changing the mouse lines and strains proved to be the most effective strategy. The United States had better equipment because they had the budget for it. For instance, they used more advanced machinery for injecting DNA solution, although the injector was actually Japanese-made and the same as what I used in Japan. Another notable difference was the microscope stand. In Japan, it was just a regular desk, but in the United States, we had one with an insulator to minimize vibrations. While the facilities were better in the United States, working overseas posed some challenges.
- *He graduated from Osaka University's Faculty of Science in 1966 and completed his doctoral program at the same university in 1971. His doctoral thesis was titled "The Ultraviolet Effect on the Physiological Functions of RNA Phage MS2." In 1972, he moved to the United States, where he conducted research on translation control of T7 phage and MS2 phage at the University of Pittsburgh. Later, he joined the National Institutes of Health (NIH) and began researching collagen genes in Benoit de Crombrugghe's lab. In 1983, he established his own research laboratory.
When you're not getting results...
In the United States, I was focused on transcriptional control research. We were investigating how transcription factors bind to promoters and regulate transcription. In our in vitro experiments, we introduced promoters and transcription factors into cultured cells. When things weren't going as planned or when results were elusive, I remember seeking advice from Professor Yamada. His Kansai dialect, as he had graduated from Osaka University, stood out. I can still vividly recall him saying, 'Well, if it's stuck to the DNA, something's bound to happen.' While it might not have been backed by a concrete scientific rationale, his words were surprisingly convincing, and I felt he was drawing from a wealth of experience. So, I decided to persevere with the experiments, believing that something must be happening. I remember adjusting my mindset and approaching the experiments with renewed determination. Eventually, the results aligned with my expectations, and I was thrilled.
Since my boss, Professor Yamada, was Japanese, there were more Japanese postdocs, and it was great to have those connections even after returning to Japan. If your fellow alumni are doing well, it serves as motivation, and it drives me to work harder, not wanting to fall behind. By putting in that effort even after returning to Japan, Professor Yamada would be pleased.
Becoming an independent associate professor and establishing a research lab
When I dedicated myself to research, I realized that there was no need to compromise.
After returning from my overseas studies, I came back to the Department of Orthopedics. Initially, the Principal Investigator was Professor Toshihiro Ochi, and later, Professor Hideki Yoshikawa took over. I had the opportunity to work with graduate students and build a team. As an assistant professor in orthopedics, I had clinical responsibilities, including outpatient clinics about twice a week, surgeries once a week, and managing the inpatient ward and being on call. During my free time, I conducted experiments with graduate students. During this period, for about three and a half years, I also worked at Osaka Police Hospital, where I focused exclusively on clinical work, primarily dealing with knee joint disorders and trauma cases.
After returning from the police hospital to the university, there was a new system established called the Independent Associate Professor System. I applied for this opportunity upon the advice of Dr. Yoshikawa and was fortunately selected. I had my own laboratory with an independent associate professor, an assistant professor, two technicians, and graduate students.
During my time as an independent associate professor, I received invaluable support and guidance from Professor Toru Nakano*, who had set up the Independent Associate Professor System. The position of an independent associate professor served as a training ground for becoming an independent researcher and lab manager. This experience allowed me to focus more on research.
When I transitioned to a research-focused role and stepped away from clinical practice, I suddenly found that I had more time to dedicate to my research. I realized that there was a distinct difference in the quality of research when I could dedicate myself to it fully, without the time constraints and compromises that came with the dual role of clinician and researcher.
- *In 1981, Professor Toru Nakano graduated from Osaka University Medical School. Afterward, He held positions as a lecturer at Kyoto University Medical School and eventually became a professor at the Graduate School of Medicine, Osaka University in 1995. From 2004 to 2022, he served as a professor in the Graduate School of Medicine and the Graduate School of Frontier Biosciences at Osaka University, leading research in the fields of pathology and epigenetics. From 2014 to 2016, he also had the honor of taking on the role of the head of the Graduate School of Frontier Biosciences, contributing to its growth and development. He was involved in establishing programs like the 'Osaka University Life Science Research Independent Apprentice Program' and dedicated his efforts to nurturing the next generation of researchers.
In this conversation, we've discussed Professor Tsumaki's journey from his time as a graduate student through his experiences studying abroad to becoming an independent associate professor. During times when clinical practice and research seemed quite distant, he shared how he managed to continue in the field of science. In the next installment, we will delve into the current landscape where clinical practice and research have grown closer together, touching upon topics like iPS cells and discussing the future.
