FBS Colloquia No.246Laboratory of Mitochondrial Dynamics
| Seminar or Lecture |
The role of ubiquitin for mitophagy in yeast SCHUSTER Ramona [Laboratory of Mitochondrial Dynamics] |
|---|---|
| Date and Time | 3 Sept. 2020 (Thu), 12:15-13:00 |
| Place | Online (Zoom) | An email will be sent with the meeting URL, ID, and password on the morning of each colloquium. |
| Language | English |
| Contact |
Koji Okamoto |
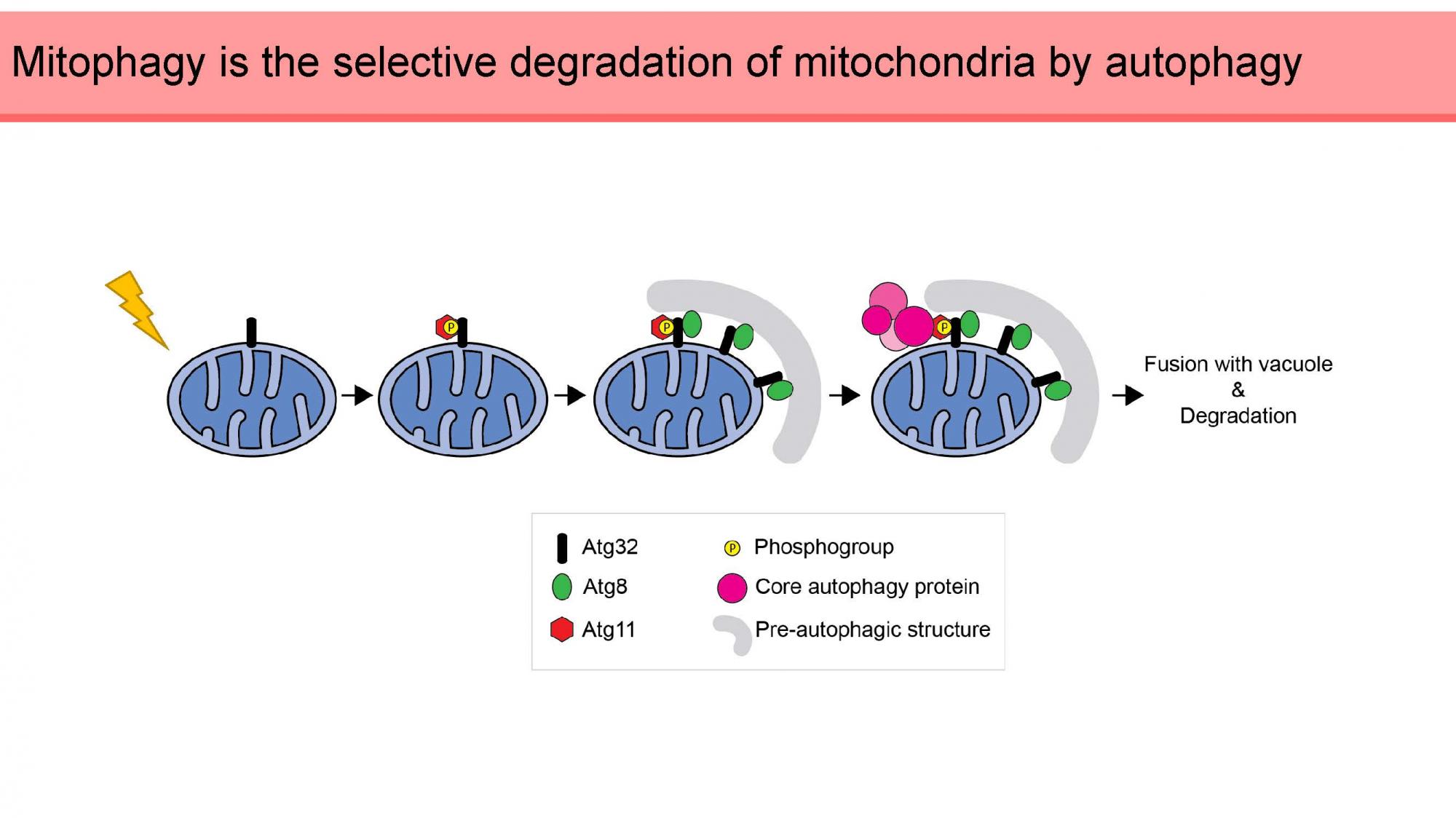
The role of ubiquitin for mitophagy in yeast
Maintenance of the cellular proteome by providing quality control of misfolded and damaged proteins as well as defective organelles is highly important for cellular viability. One way to dispose of aberrant proteins is via the ubiquitin-proteasome system which degrades proteins that are specifically labeled for degradation with a small, conserved protein called ubiquitin. Ubiquitin is attached to substrates by an enzymatic cascade involving several steps it can be cleaved off of substrates by ubiquitin-specific proteases (DUBs). Furthermore, the lysosome, or vacuole in yeast, degrades aggregated proteins but also whole organelles by a pathway called autophagy. One selective branch of the autophagy pathway is the degradation of mitochondria, called mitophagy. While in mammalian cells, a ubiquitin-dependent mitophagy pathway has been described, no such process has yet been reported for yeast. Here, we systematically analyze all 21 non-essential DUBs in yeast for their role in selective autophagy, particularly mitophagy. In this colloquium, I will present to you our preliminary data, showing that several DUBs de- or increase the efficiency of mitophagy and affect the levels of the yeast mitophagy receptor Atg32 as well as its role in mitophagy initiation. This work will help elucidating the connection between the ubiquitin-proteasome system and mitophagy in yeast.