FBS Colloquia No.325JEOL YOKOGUSHI Research Alliance Laboratories
| Seminar or Lecture |
Assembly mechanism of the polypeptide channel complex of the bacterial flagellar protein export apparatus and report on the current status of CRYO-FIB-SEM (JIB-4700F) Miki Kinoshita [Specially Appointed Assistant Professor, JEOL YOKOGUSHI Research Alliance Laboratories] |
|---|---|
| Date and Time | 23 May 2023 (Tue), 12:15~13:00 |
| Place | Online (Zoom) | An email will be sent with the meeting URL, ID, and password to all FBS members. |
| Language | Japanese |
| Contact |
Tomoko Miyata (Specially Appointed Associate Professor) |
Assembly mechanism of the polypeptide channel complex of the bacterial flagellar protein export apparatus and report on the current status of CRYO-FIB-SEM (JIB-4700F)
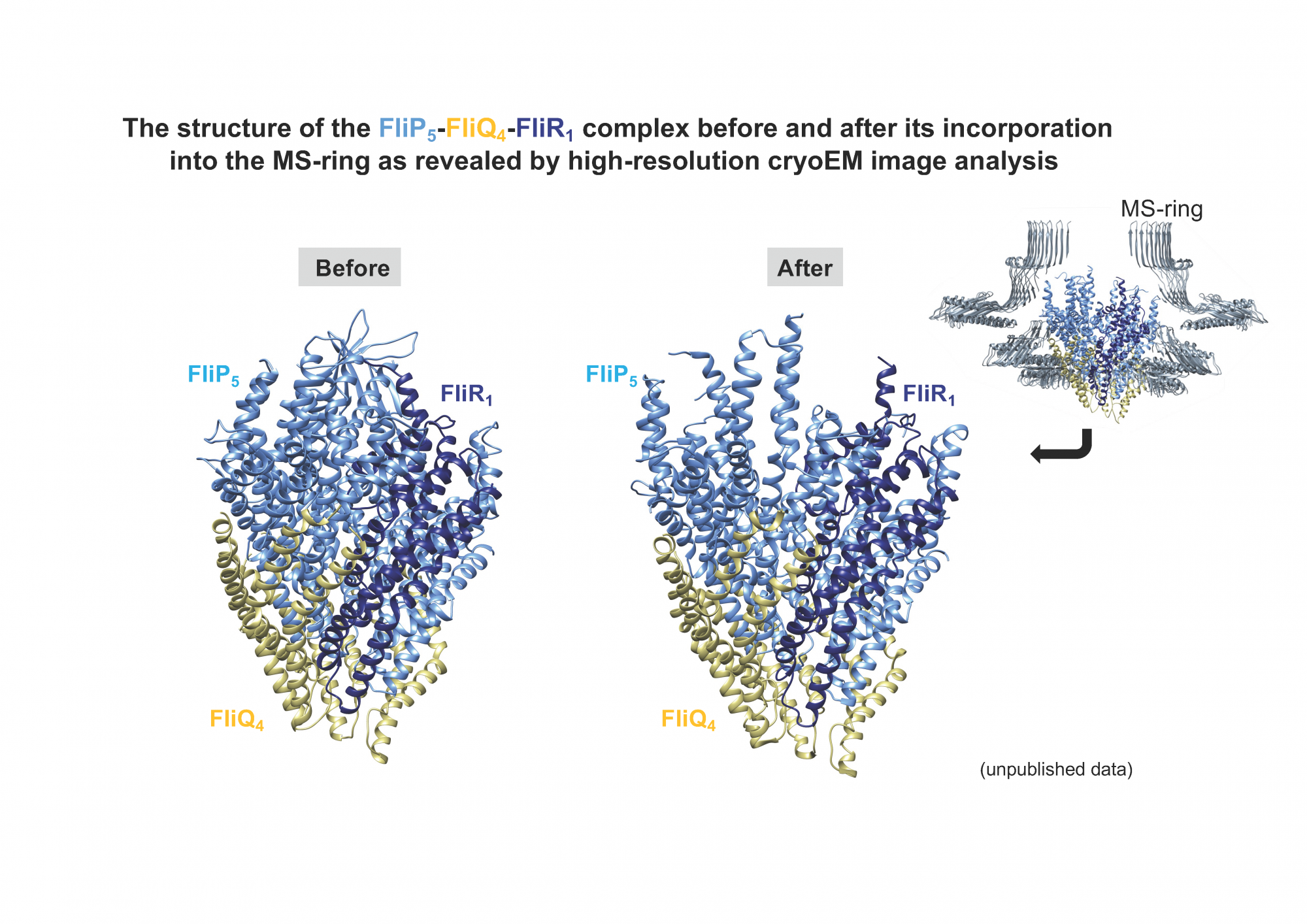
The bacterial flagellum is a supramolecular motility machine consisting of at least three distinct functional parts: the basal body that acts as a rotary motor, the filament that functions as a helical propeller to produce propulsion; and the hook that works as a universal joint that transmits torque produced by the motor to the helical propellor. To construct the flagellum on the bacterial cell surface, flagellar structural subunits pass the cytoplasmic membrane via the flagellar protein export apparatus located at the base of each flagellum, diffuses through a narrow central channel, and assemble at the distal end of the growing flagellar structure. Three transmembrane export gate proteins, FliP, FliQ, and FliR, form a polypeptide channel complex with a stoichiometry of 5 FliP, 4 FliQ, and 1 FliR, allowing flagellar structural subunits to be translocated across the cytoplasmic membrane. The FliP5-FliQ4-FliR1 complex is formed by a helical arrangement of subunits within a central pore of the basal body MS-ring. In this colloquium, I will present the structure of the FliP5-FliQ4-FliR1 complex before and after its incorporation into the MS-ring as revealed by high-resolution cryoEM image analysis and discuss the mechanism of export channel formation inside the flagellum. The modification of the cryo-FIB-SEM (JIB-4700F), which is being carried out in cooperation with JEOL, is almost completed and is now in operation, allowing us to collect a complete set of tomographic data on cell and tissue sections using the CRYO ARM-300. Therefore, I will also report on the current status of the cryo-FIB-SEM.