FBS Colloquia No.271Laboratory for Ultra-High Magnetic Field NMR Spectroscopy, Research Center for Next-Generation Protein Sciences, Institute for protein research
| Seminar or Lecture |
Structural analysis of the rotor and stator complex in bacterial flagellar motor using nuclear magnetic resonance and cryogenic electron microscopy in institute for protein research Tatsuro NISHIKINO [Postdoctoral Associate, Laboratory for Ultra-High Magnetic Field NMR Spectroscopy, Research Center for Next-Generation Protein Sciences, Institute for protein research] |
|---|---|
| Date and Time | 17 Jun. (Thu) 2021, 12:15-13:00 |
| Place | Online (Zoom) | An email will be sent with the meeting URL, ID, and password to all FBS members. |
| Language | Japanese |
| Contact |
Yohei Miyanoiri |
Structural analysis of the rotor and stator complex in bacterial flagellar motor using nuclear magnetic resonance and cryogenic electron microscopy in institute for protein research
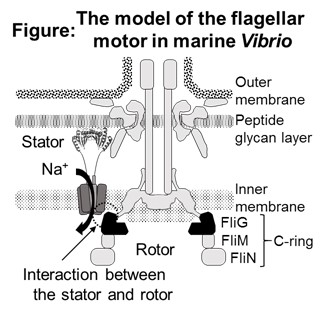
Many bacteria navigate using rotation of a helical flagellum. The torque of the flagellar motor is generated by interaction between the rotor component, FliG, and the stator complex, depending on the driving ion (Figure). This process converts the energy of electrochemical gradient across the cell membrane into flagellar rotation. Although the motor can rotate clockwise and counterclockwise rotation, the molecular mechanism of energy conversion and determination of the rotation via interaction between the rotor and stator is still unclear.
In this study, I would like to present the structural dynamics study of Vibrio FliG using nuclear magnetic resonance (NMR) and the structure analysis of Aquifex MotA, one of the stator components, by cryogenic electron microscopy. These advanced facilities can be used through a joint usage program of institute for protein research (IPR).