Asymmetrical localization of Nup107-160 subcomplex components within the nuclear pore complex in fission yeast
| Journal | PLoS Genet. 15(6):e1008061 (2019) |
|---|---|
| Title | Asymmetrical localization of Nup107-160 subcomplex components within the nuclear pore complex in fission yeast |
| Laboratory | Nuclear Dynamics Group |
Abstract
The nuclear pore complex (NPC) forms a gateway for nucleocytoplasmic transport. The outer ring protein complex of the NPC (the Nup107-160 subcomplex in humans) is a key component for building the NPC. Nup107-160 subcomplexes are believed to be symmetrically localized on the nuclear and cytoplasmic sides of the NPC. However, in s. pombe immunoelectron and fluorescence microscopic analyses revealed that the homologous components of the human Nup107-160 subcomplex had an asymmetrical localization: constituent proteins spNup132 and spNup107 were present only on the nuclear side (designated the spNup132 subcomplex), while spNup131, spNup120, spNup85, spNup96, spNup37, spEly5 and spSeh1 were localized only on the cytoplasmic side (designated the spNup120 subcomplex), suggesting the complex was split into two pieces at the interface between spNup96 and spNup107. This contrasts with the symmetrical localization reported in other organisms. Fusion of spNup96 (cytoplasmic localization) with spNup107 (nuclear localization) caused cytoplasmic relocalization of spNup107. In this strain, half of the spNup132 proteins, which interact with spNup107, changed their localization to the cytoplasmic side of the NPC, leading to defects in mitotic and meiotic progression similar to an spNup132 deletion strain. These observations suggest the asymmetrical localization of the outer ring spNup132 and spNup120 subcomplexes of the NPC is necessary for normal cell cycle progression in fission yeast.
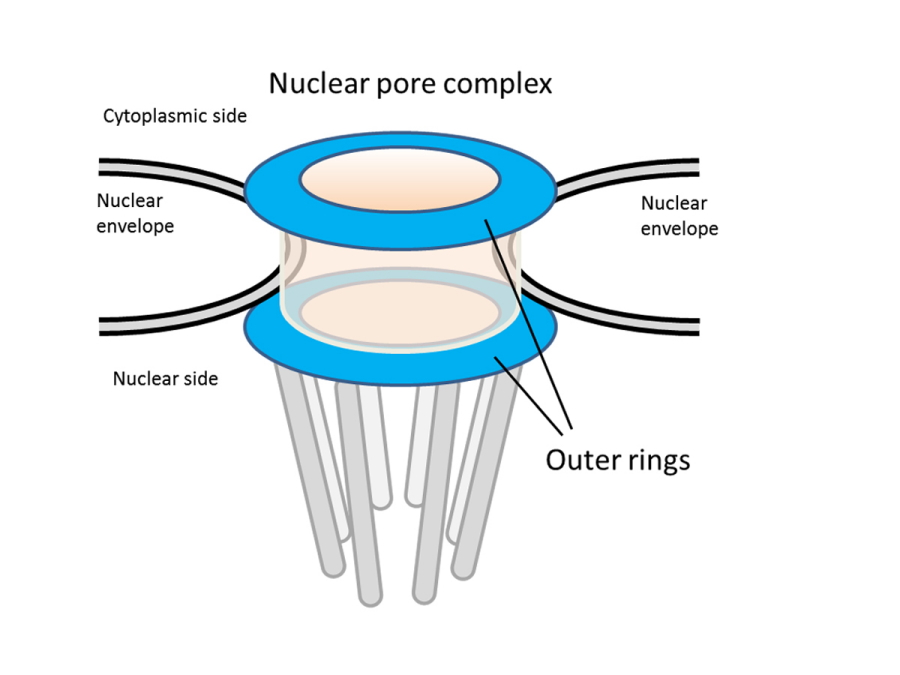
Fig. 1. Schematic of the nuclear pore complex.
The nuclear pore complex is an eight-fold rotational symmetric cylindrical structure penetrating the nuclear membrane. Molecular transport between the nucleus and the cytoplasm takes place by passage of molecules through the interior of the tubular structure. The outer ring structure is a ring-like structure which is found on both the inner and cytoplasmic sides of the nuclear pore complex in humans and budding yeast.
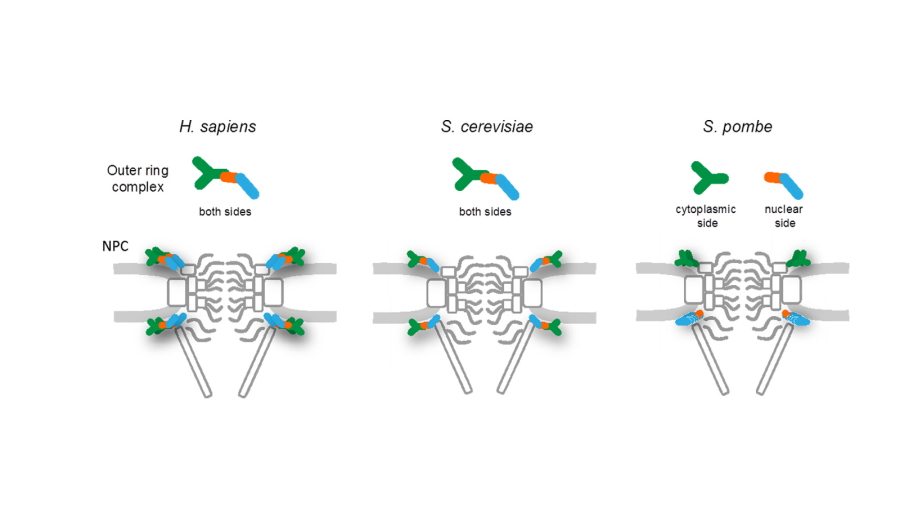
Fig. 2. Schematic drawings of the outer ring complex and the NPC structure in H. sapiens (left) and S. cerevisiae (middle) in comparison to that in S. pombe (right).
| Authors | Haruhiko Asakawa (1), Tomoko Kojidani (2, 3), Hui-Ju Yang (1), Chizuru Ohtsuki (1), Hiroko Osakada (2), Atsushi Matsuda (2), Masaaki Iwamoto (2), Yuji Chikashige (2), Koji Nagao (4, 5), Chikashi Obuse (4, 5), Yasushi Hiraoka (1, 2), Tokuko Haraguchi (1, 2)
|
|---|---|
| PubMed | 31170156 |
