
Structural study of the flagellar motor
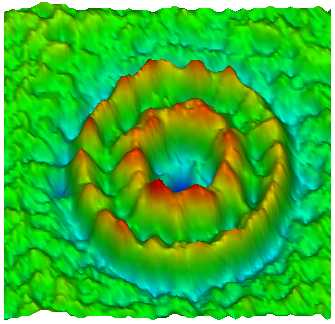
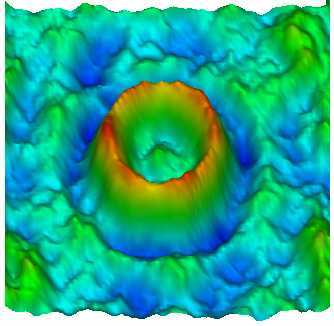
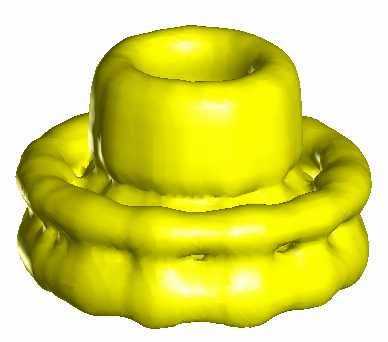
In spite of long-time studies by many scientists, still little is known about the mechanism of the flagellar motor, as compared with the other bio-molecular motors. Detailed research of this nanomachine was hampered by difficulty of sample preparation suitable for structural and functional analyses. The FliF ring complex, the basal component of the rotor, is one of the most important target of such analyses, but it is also one of the most troublesome specimen to handle because of its hydrophobic property.
We established a preparation method for the FliF ring complex in either monodispersed form or 2D crystalline array, and carried out a preliminary structure analysis by electron microscopy (1). The results showed some structural change in the central channel, the export pathway for flagellar proteins, which suggested the presense of open and closed states of the gate. Currently, we are working on a more detailed structure analysis using an electron cryomicroscope, Hitachi EF-2000, which is equiped with a cold field emmission electron source and a energy filter. We also work on in vitro assembly of this complex with other proteins such as FliG, which is involved directly in the motor rotation.
(1) Suzuki, H., Yonekura, K., Murata, K., Hirai, T., Oosawa, K. & Namba, K. (1998) A structural feature in the central channel of the bacterial flagellar FliF ring complex is implicated in type III protein export. J. Struct. Biol. 124, 104-114. [PubMed] [J. Struct. Biol.]


