
NanoAssembly Group
Overview
X-ray crystallography and fiber diffraction are used to analyze the structures of the flagellar axial assembly and motor complex. Of nine proteins that form the flagellar axial structure, four have been crystallized by truncating both terminal regions that stabilize the polymer form and three structures have been solved at around 2 Å resolution.
<Major results>
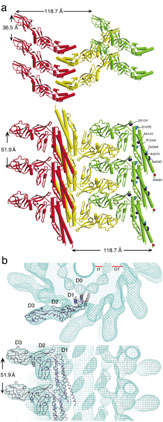

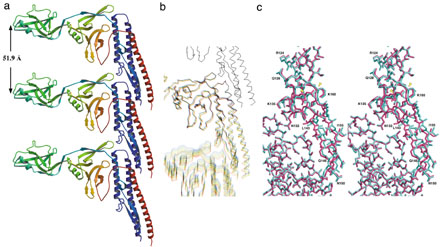


The mechanism of flagellar supercoiling to function as a helical propeller is the presence of two protofilaments with two distinct repeats distances and lateral packing modes in the 11 protofilaments that form the tubular structure of the filament. One repeat is 52.7 Å and the other is 51.9 Å. The crystal structure of a 41-kDa flagellin fragment revealed the structure of protofilament with the shorter repeat distance, from which we identified the β-hairpin in domain D1, which forms the axial contact, as the lengthwise switch unit.
Publications
1
Suzuki, H.; Yonekura, K.; Murata, K.; Hirai, T.; Oosawa, K.; Namba, K. (1998)
A structural feature in the central channel of the bacterial flagella FliF ring complex is implicated in Type III protein export. J. Struct. Biol. 124, 104-114.
2
Samatey, F.A.; Imada, K.; Vonderviszt, F.; Shirakihara, Y.; Namba, K. (2000)
Crystallization of the F41 fragment of flagellin and data collection from extremely thin crystals. J. Struct. Biol. 132, 106-111
3
Samatey, F.A.; Imada, K.; Nagashima, S.; Vonderviszt, F.; Kumasaka, T.; Yamamoto, M.; Namba, K. (2001)
Structure of the bacterial fragellar protofilament and implications for a switch with sub-Å precision. Nature. 410, 331-337 .
Namba Protonic NanoMachine Project, ERATO
Home








